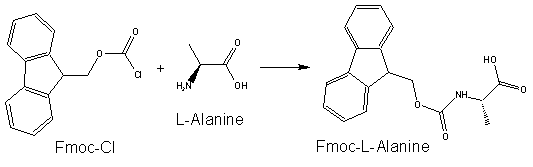
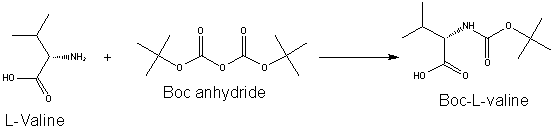
Protective Groups for Peptide SynthesisBecause of amino acid is an acid with a basic group at one end and an acid group at the other, polymerization of amino acids is common in reactions where each amino acid is not protected. In order to prevent this polymerization, protective groups are used. Currently, two protecting groups are commonly used in solid-phase peptide synthesis – Fmoc (9-fluorenylmethyl carbamate) and t-Boc (Di-tert-butyl dicarbonate). Fmoc Protecting GroupThe use of Fmoc chemistry for protection of the alpha amino group has become the preferred method for most contemporary solid and solution phase peptide synthetic processes. Fmoc has also been shown to be more reliable and produce higher quality peptides than Boc chemistry. The advantage of Fmoc is that it is cleaved under very mild basic conditions (e.g. piperidine), but stable under acidic conditions. After base treatment, the nascent peptide is typically washed and then a mixture including an activated amino acid and coupling co-reagents is placed in contact with the nascent peptide to couple the next amino acid. After coupling, non-coupled reagents can be washed away and then the protecting group on the N-terminus of the nascent peptide can be removed, allowing additional amino acids or peptide material to be added to the nascent peptide in a similar fashion. Boc Protecting GroupBefore the Fmoc group became popular, the t-Boc group was commonly used for protecting the terminal amine of the peptide, requiring the use of more acid stable groups for side chain protection in orthogonal strategies. Boc groups can be added to amino acids with Di-tert-butyl dicarbonate (Boc anhydride) and a suitable base. The t-Boc protecting group is removed by exposing the Boc-protected residue on the chain to a strong acid. Typical reagents of choice for deprotection in existing methods are trifluoroacetic acid (TFA) in dichloromethane, hydrochloric acid or methanesulfonic acid in dioxane. The acid used to remove the Boc protecting group is typically neutralized with a tertiary amine such as N-methylmorpholine, N-diisopropylethylamine (DIEA) or triethylamine (TEA). |
||
|
||